Laminate Medical Technologies rolled out its FDA pivotal trial of the VasQ device
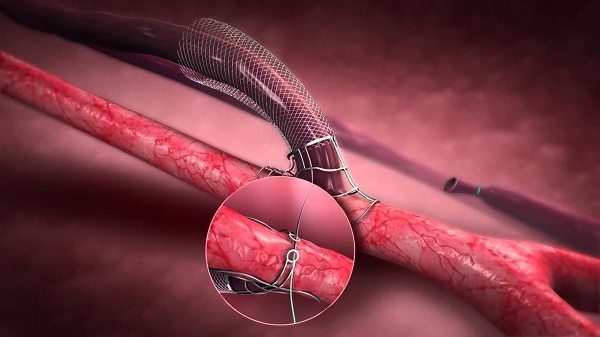
Privately-held start-up developing VasQ, Laminate Medical Technologies declared the roll- out of its FDA pivotal study in the US. VasQ is an implanted blood vessel external support device for patients requiring arteriovenous fistula as vascular access for hemodialysis. VasQ is mandated for patients suffering from kidney failure and in need of dialysis, which requires vascular access. VasQ is an external scaffold placed over the fistula, creating an adequate geometric configuration with the artery and lowering the tension in the vein. This allows proper blood flow during dialysis while reducing vein blockage created by thickening of the vein wall. Studies reflect immense success rate of VasQ which is a CE Marked device already in use in hospitals in Europe and in Israel, displaying commendable outcomes.
Dr. Jason Burgess who performed the first surgery at the Carolinas Medical Center-Mercy, Charlotte, North Carolina expressed “We are excited to be the first site that enrolled patients in the US. The device was easy to implant and I am happy with the surgical results. We are looking forward to evaluating the device’s long term effect.”
Prospective, multi- center, single-arm, open-label, 13-site study is set to enlist 129 male and female patients, 18 to 80 years old, who require creation of new brachiocephalic fistula. During this screening, surgeons are set to assess additional eligibility criteria such as adequate blood vessel anatomy and absence of potentially precluding past and current medical conditions and comorbidities. The primary effectiveness endpoint for this trial will be the primary patency rate 6 months after creation of the arteriovenous fistula. Patients will be followed for a total of 2 years.
Image Source: Laminate Medical Technologies