Tusker Medical enlists first patient for OTTER Study
Tusker Medical announced the enrollment of first- in-office patients in the OTTER Study implied for Tusker’s TULA (Tubes Under Local Anesthesia) System. Via an approved Investigational Device Exemption (IDE), OTTER Study aims to assess the in-office placement of tympanostomy tubes in approximately 300 children between 6 months and 12 years old enrolled at up to 25 investigational sites in the United States and Canada.
Tympanostomy tube insertion is the most common ambulatory pediatric surgery in the United States, performed to treat recurrent ear infections or persistent fluid in the middle ear.
Larry Lustig, MD, principal investigator and Chair of the Department of Otolaryngology-Head & Neck Surgery at the Columbia UniversityMedical Center in New York City said “While general anesthesia for tube procedures is considered quite safe, there is an emerging body of research suggesting that there may be longer-term neurodevelopmental risks associated with pediatric general anesthesia. The OTTER Study will investigate a new device/drug combination product designed to enable in-office tube procedures in pediatric patients, avoiding the risks of general anesthesia.”
Amir Abolfathi, President and CEO of Tusker Medical said “This provides an alternative to phenol (a denaturing acid), ear canal injections, or other local anesthesia methods, none of which have FDA approval for pediatric tympanic membrane anesthesia and are often painful or take an unacceptably long time to achieve anesthesia.”
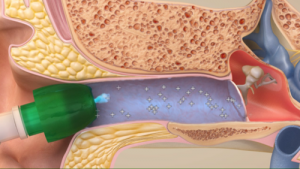
Once the tympanic membrane is numb, the Tube Delivery System is used to insert the tympanostomy tube. With a single button push, the Tube Delivery System automatically creates the myringotomy and inserts the tube in less than 500 milliseconds. The myringotomy cutter is exposed only for the very brief moment when the myringotomy is created, and is otherwise recessed within the device, an important safety feature when performing procedures in an awake child. Sedatives or anxiolytics such as midazolam or nitrous oxide are not permitted in the OTTER study.

